New Technologies in Protective Coatings
Edited by Carlos Giudice and Guadalupe Canosa
Abstract
Fire retardant coatings are often required to protect a wide range of products of both flammable and nonflammable against fire. It is an oldest, most efficient, and easiest method to apply any surface without modifying the intrinsic properties of materials. Moreover, the initial phase of fire always occurs on the surface by ignition, and hence, it is important to concentrate on the surface protection of a material. Being an organic nature of conventional surface coating will burn easily and generate smoke and toxic fumes, which may not be suitable for application where fire protection or fire prevention is required. Reaction-to-fire and/or resistance-to-fire are to be considered for assessing both flammable and non-flammable material by using fire retardant and fire resistant or fire protective coatings. The degree of fire retardation mainly depends on the coating thickness, substrates, and efficiency of formulations. This chapter explains briefly the fire retardation of wood by using fire retardant coatings.
Keywords
- fire retardant
- wood
- intumescent coating
- charring
- nanocomposites
- sol-gel process
1. Introduction
The devastating nature of fire creates havoc that result in great loss of both lives and assets. It can also cause serious human sufferings and financial losses. Thus, great attention has been paid to develop the effective fire protection methods in order to prevent any casualties and reducing economic fire damage to an acceptable level. Nowadays, because of rapid changes in building and developments in architectural technology have led to more complex structure of which using a strong and lightweight components, the wide range of materials in the form of organic or inorganic and synthetic or natural used in building are easily flammable or readily burning on exposure to high temperatures of fire. On the other hand, non-combustible material such as metals (except aluminum and magnesium) and concrete will not burn and support the combustion, or release the flammable vapors when subject to fire. However, it is important to consider these materials can withstand a fire for a specified period of time. In this regard, the strength and deformation of these materials deteriorate significantly at high temperature. As a result, the structural elements and assemblies may deform or even collapse when exposed to fire conditions. The allowable or extra time in a fire situation depends largely on the anticipated temperature development of the fire, which depends on the type and amount of combustible materials present and the ventilation condition [1–3]. Although protection of materials against fire by the use of coatings for indefinite periods is impossible, it can delay the spread of fire or keep a structure intact against fire, thereby allowing sufficient time for safety measures to be taken. This chapter covers with a discussion of the fire retardation of wood products that can be available in scientific literatures.
2. Fire retardant vs. fire resistant
There is always misconception about inhibition of fire by using coating of either fire retardant or fire resistance. Figure 1 shows the development of fire in a compartment, which distinguishes the usage of coating in a particular stage of fire. For instance, the material properties play an important role in prior to flashover, which will be controlled by fire retardant coatings (ignition, flame spread, release of heat, smoke, and gases), whereas fire resistant coatings are mainly predominated in protection of structures that occur after flashover, that is, phase of fully developed fire. Post-flashover fires (temperature above 600°C) are considered as hazardous fires for structures [4]. Fire retardant coatings are mainly involved for reaction-to-fire to retard or inhibit the combustion of flammable materials (wood, foam, textile fabrics, electric cables, and fiber reinforced composites) whereas fire resistant or fire protective coating for resistance-to-fire to protect the non-flammable materials. Different test parameters, such as oxygen index (OI), flame spread rate, ignition time, heat intensity, smoke generation, and release of toxic gases, are to be considered for assessing the flammable materials. On the other hand, thermal insulation, integrity, and load-bearing capacity are paramount important for the fire resistance of non-flammable materials. Depending on the types of material and intended application, specific fire performance properties of building materials are to be tested by use of different test methods. The relevant test standards for both coatings are different that also depend on the region of countries.
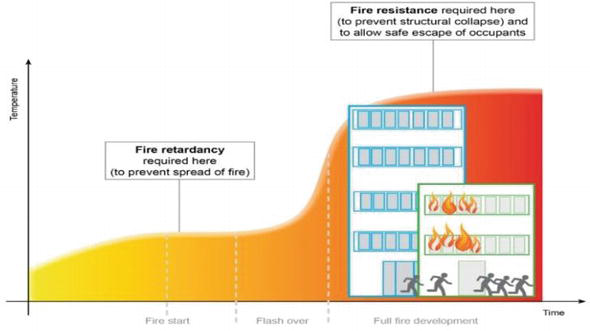
Figure 1.
Phases of fire development in an enclosure.
In general, conventional organic surface coating is easily ignitable, melts, drips, and it may cause severe injury and damage to the substrates in the event of fire. Therefore, coatings that are designed to formulate should not contribute a significant amount of fuel to the fire and, at the same time, limit the flame spread and smoke development. Fire retardant coating is one of the easiest, oldest, and most efficient ways to protect the materials against fire. This approach does not cause chemical modification of the substrate, but rather the formation of a protective layer which alters the heat flux to the substrate and can inhibit its thermal degradation, ignition, or combustion [5]. The ideal fire retardant coatings should have minimum flame spread, negligible or low release of smoke and/or toxic gases, be easy to apply, show good wear resistance, adhere to the underlying substrate and offer low cost. Typically, they are based on chlorinated alkyds or brominated epoxy resin and filled aluminum hydroxide or a combination of chlorinated paraffin and antimony oxide system. However, the wide range of flame retardants in the form of reactive or additive and different classes (halogen, phosphorus, nitrogen, minerals, oxides, intumescent, and nanofillers) are available commercially that are incorporated into coating formulations to inhibit or retard the burning of materials. The selection of flame retardants can be tailor-made for a specific polymer binder in coating formulations. Fire retardant coatings look like architectural paints and mainly available in solvent form and are applied by conventional methods, brush, roller, and spray.
Fire retardant coatings can be classified into two groups: non-intumescent and intumescent coatings [6]. Non-intumescent coatings are basically decorative, architectural coatings that contain flame retardant additives designed to reduce the rate of flame spread and smoke development of combustible substrates. They are rated as Class A, B or C based on the ability to not contribute to fire and smoke. Rate of flame spread depends on both substrate and thickness of the film. On the other hand, intumescent coatings swell under the influence of heat to form a multicellular charred layer, which acts as an insulating barrier and slows heat and mass transfer between the condensed and vapor phases. This intumesced char can increase to up to 50 times the original thickness of the applied coating. Two types of fire retardant coating either pigmented/colored or clear, transparent varnish are available on the market that are designed for use on different materials and that respond very differently when exposed to fire. They are mainly used in construction, transport, wall and ceiling linings, and other areas require products to satisfy the requirement of classes.
The burning of surface coating is believed to proceed by a free radical mechanism where both ˙H and ˙OH radicals are chain carriers and take part in a number of reactions in the flame zone. The function of fire retardant coatings is to protect the substrate that depends on the mode of operation of flame retardants [7]. For example, the coatings formulated with halogenated compounds are more effective in gas phase, and they act in the flame zone by forming a blanket of halogen vapor that interferes with the propagation of the flame by interrupting the generation of highly reactive free radicals, thus helping flame extinguishments. However, the release of toxic and corrosive gases from halogenated compounds while burning is ecologically unsafe. The action of phosphorus in coatings varies with the type of flame retardants and the polymer binders. They mostly operate in condensed phase to form a protective char layer that acts as physical barrier to heat transfer from the flame to the substrate and to diffusion of gases. The vapor phase action is also found to be effective in phosphorus flame retardants, which are capable of controlling the high energy radicals in the flame. Intumescent flame retardant is a combination of an acid source, a char former and a gas source, and sometimes, it is available in a single compound including all three functions. The mechanism of intumescent coatings is to undergo an endothermic decomposition reaction at an elevated temperature that causes the coating to swell and form into a highly porous, thick, and thermally stable char layer that has a very low thermal conductivity (heat insulation). Other flame retardants such as metal oxides and hydroxides are operated by cooling with the release of water and by diluting or removing the flammable fuels and oxygen.
4. Fire retardancy of flammable materials
Typically, the flammable materials are easily combustible and rapidly growing in a fire (reaction-to-fire), in terms of the spread of fire or propagation of fire, up the stage when flashover occurs in a compartment. Flashover can occur quickly in seconds or slowly depending on the speed of fire growth rate. Wood is the most frequently used combustible products in addition with polymers (plastics and rubbers), foams, textiles, cables, and fire reinforced composites. Three different kinds of methods, such as clear or transparent varnish paints, pigmented intumescent reactive coatings, and surface impregnations are utilized in wood to enable the restriction of growth and/or spread of fire. The European fire classification for reaction-to-fire is based on fire growth rate index (FIGRA), which indicates the time to reach flashover in the standardized reference test as per BS EN 14390. Other test methods, the single burning item (SBI) test (BS EN 13823; 2002), radiant panel test for flooring (EN ISO 9239-1; 2002) and either the small flame test (BS EN 11925-2; 2002) or the bomb calorimeter (BS EN ISO 1716), are also relevant to the fire retardant system in Euro-class [8]. Two standards, such as fire propagation test (BS476, part 6:1989) and surface spread of flame test (BS476, part 7:1987), were applicable to the fire test methods in the UK for flammable materials. The BS476, part 6 test method is intended to provide a comparative measure of the contribution to the growth of fire of a product. The test result is expressed as fire propagation index (I) and three sub-indices, i1, i2, and i3. The higher the fire propagation index means the greater the growth of fire. On the other hand, the BS476, part 7 measures the lateral spread of flame along the surface of a specimen, which is mounted at right angles to a high intensity radiation panel. The extent and rate of flame spread of specimen are used to determine the classification, which can range from Class 1 (the best) down to Class 4. The ASTM E 84, 2010 and ISO 5660 are most commonly used in USA and International standards for testing of flammable materials. The factors influencing the performance of coating systems are thickness, density, substrates, composites, and panel type. Each type of product shall be evaluated and representative specimens shall be tested to ensure that the effect of each variable parameter is considered.
5. Wood
Wood is one of the most versatile, sustainable, aesthetically pleasing, and environmentally benign materials. It can be classified into hard and soft wood, which can have different percentages of cellulose, hemicellulose, and lignin. Different types of wood products including solid wood-based panels (particleboard, hardboard, fiberboard, fir Douglas plywood), structural timbers, glued laminated timbers, cladding and wood floorings are widely used for structural purposes in building construction, flooring and furnishing materials that found in homes, schools, and offices around the world. The basic deficiencies of wood products are flammability, poor dimensional stability, and low resistance to micro-biological decay that must be addressed when used as a construction material. Because of easily flammable and contribute fuel to fires, wood is considered to poor construction materials. The flaming combustion of wood is mostly supported by cellulose [9].
The various categories of action are typically described in wood treated with flame retardants: (1) an acceleration of dehydration and carbonization that provides thermal insulation, (2) chemical modification of wood pyrolysis, (3) absorb the surrounding heat by endothermic reactions, (4) inhibition of the flaming combustion in the gas phase, and (5) increase the thermal conductivity of wood in order to dissipate the heat from the wood surface [10]. The fire retardation of wood is typically treated with flame retardant chemicals that are coated onto the surface of wood by painting, spraying or dipping methods and/or impregnated into the wood structure using vacuum pressure technique or plasma treatments [11], which inhibits ignition and do not contribute to the spread of flame. Three components of wood have quite different decomposition range; for instance, temperature from 200 to 260°C is for hemicellulose, temperature from 240 to 350°C is for cellulose, and temperature from 280 to 500°C is for lignin. When heated, wood undergoes degradation and combustion to produce volatile gases, tars (levoglucosan), and carbonaceous chars. The fire performance of wood-based products and test methods has been reviewed and studied extensively [12–22]. Traditional flame retardants, such as boron compounds, mineral acids, and inorganic salts, (monoammonium phosphate, diammonium phosphate, guanylurea phosphate, guanidine phosphate, ammonium polyphosphate, and melamine phosphate), may considerably improve the fire retardant properties of wood [23]. However, the use of boron and formaldehyde-based systems is likely to be declined in accordance with the growing awareness of environmental issues and consumer safety. In addition, inorganic salts may also affect the performance of wood in various ways by increasing hygroscopicity, reducing strength that leads to dimensional instability, wood degradation, corrosion of metal fasteners, adhesion problems, and increased abrasiveness. The flame retardants based on phosphorus, nitrogen, silicone, and char forming additives are still prominent solution to address the flammability and environmental issues [24].
Cone calorimeter is a most commonly used bench-scale method to evaluate the flammability of wood. Shi and Chew have investigated the carbon monoxide (CO) yield of six species of wood samples under different external heat fluxes and moisture content by spontaneous ignition in a cone calorimeter. Spontaneous ignition is a complex phenomenon that combustible materials are ignited by internal heating, without the spark plug. As compare to piloted ignition, process of spontaneous ignition is much closer to the development of real fire. Results observed that thickness of wood has little effect to peak CO release rate, but the time to peak is postponed with a higher thickness. The peak CO release rate decreases with a higher external heat flux, but the decrease is not obvious when heat flux increases from 50 to 75 kW/m2. Average CO yield is inversely proportional to external heat flux, thickness, and density. They concluded that both flame and moisture can also reduce CO release rate because energy used for water evaporation increases with high moisture content [25]. The effect of variable heat flux and oxygen concentrations (20.9, 18, 16 and 15%) on ignition time and mass loss rate of wood was investigated to obtain the kinetic parameters, activation energy and frequency factor. It was found that with increasing the oxygen concentration, the mass loss rate was increased, but the ignition time, the activation energy and the frequency factor were decreased [26]. Critical heat flux for ignition has been calculated to be between 10 and 13 kW/m2 for a range of wood products. Density, thickness and moisture content have a large influence on the material dependent properties [27].
6. Charring rate of wood
The charring rate, speed at which charring depth advance in the material when exposed to high temperature, is a critical parameter for flammability of wooden samples because it allows the determination of the size of the residual section of wood. It depends on wood species, density, moisture content, permeability, composition and direction of burning [28]. For example, the charring rate of flame retardant-treated wood is linearly proportional to the applied heat flux in cone calorimeter and inversely proportional to the density of wood. Charred wood is bounded by the transition between the pyrolysis layer, the zone where thermal degradation of wood and char formation is actually occurring and the char layer, a zone of cracked charcoal that has no relevant strength or stiffness properties. Charring depth is the distance between the outer surface of the original member and the position of the char line. The base of char layer is widely occurs between 280 and 300°C. Beikircher et al. determined the charring rate, mass loss and temperature development of Norway wood coated with transparent and colored intumescent coatings using cone calorimeter and ISO 834 furnace test. They found that the intumescent coatings reduce the charring rate significantly at all irradiance and cellulosic fire exposure condition (ISO 834 test curve) in comparison with the uncoated (REF) wood sample (Figure 2). Intumescent coatings can delay the onset of charring and reduce the charring rate of wood [29].
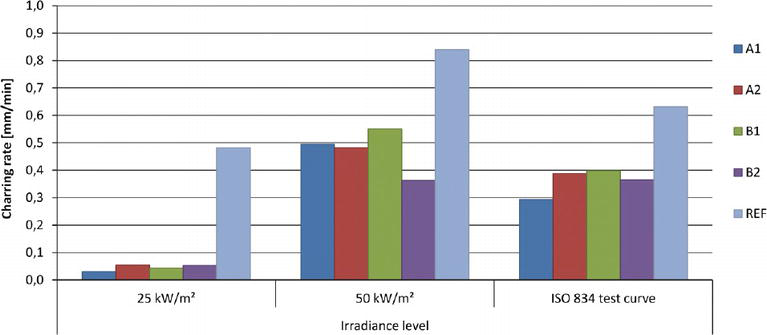
Figure 2.
Charring rate of intumescent coated and uncoated wood exposed to different test conditions [29].
7. Boron-based fire retardant coatings
Boric acid (H3BO3) and borax (Na2B4O7.10H2O) are used as borates, which are water soluble and most commonly used flame retardants in wood products. Boron-containing formulations are also used to improve the service life of timbers, in terms of both increase in resistance to biological attacks and renders more resistant to burning. Atar and Keskin studied the flammability of varnish coated Uludag Fir wood and boron compound impregnation. The ASTM D1413-99 standard is used to impregnate the wood by vacuum technique using a mixture of boric acid and borax. The flammability of wood was characterized by ASTM E160-50. They found that boron impregnations showed decrease the flammability of wood as compared to varnish coated wood. It was suggested that the impregnation of wood with boron compounds before varnish coating can decrease the combustion temperature and provide security to wood structure. Further, they investigated the impact of boron compounds impregnation on combustion properties of the laminated veneer lumber obtained from European oak and Lombardy poplar woods. Because of the interaction of impregnation materials with wood structure, the lowest flame source combustion was observed in treated wood [30, 31].
Blasi et al. have investigated the pyrolysis products of fir wood impregnated with boric acid at heating temperatures of 377 and 527°C. It was found that the yields of char and water increase with boric acid concentration (below 2%), and the amounts of organic liquid products are reduced. The boric acid treatment lowers the activation energy and delayed the most important oxidation reaction of fir wood. The reaction temperature does not affect the pyrolysis product distribution but at lower temperature, the higher the char yields, water and non-flammable volatile products were observed [32]. Further, the effect of diammonium phosphate (DAP) and diammonium sulfate (DAS) treatment on the pyrolysis of wood was investigated. Two ammonium salts are widely used as flame retardants in wood substrate, and both are significantly alter the char reactivity. A lower activation energy and a higher reaction order are obtained for DAP-treated sample as compared to wood treated with DAS [33]. Both treatments produce an equal amount and composition of solid, liquid and gaseous products during decomposition. However, with increasing the concentration of salts and/or decreasing the heating temperature produce greater amount of char and water. It was concluded that DAP treatment showed better flame retardancy on the basis of the formation of higher yields of char, water and lower combustible flammable liquids. It also confirmed that the release of decomposed volatile products depends on the DAP concentration [34, 35]. The thermogravimetric analysis was carried out in air of wood and wood impregnated with DAP concentrations range up to 20% and heating rates between 5 and 20°C/min. Results showed a three step decompositions in sequence of wood decomposition, induction and char oxidation, and concluded that the estimated kinetic parameters are independent of the heating rate but vary with the DAP concentration. However, the activation energies of the various steps remain practically constant except for the decomposition of the cellulose component or the decomposition step, depending on the complexity of the mechanism [36].
The fire performance of Douglas fir wood was studied by both natural extractives and a mixture of boric acid and borax treatment. Dual treatments of wood with the natural extractives and borates were targeted to benefit from their potential cumulative protections, which are biological resistance and fire retardancy. It was observed that both treated wood specimens showed excellent fire retardant performance [37]. Tomark and Cavdar studied the effect of boron powder (BP), mixture of boric acid (BA) and borax (BX) and flame retardant agent (FA) based on liquid blend of limestone and silicone oil (SO) treatment on the oxygen index (OI) of Scots pine wood of bare and after leaching process. Leaching procedure was carried out to determine the permanent performance of the preservatives in wood. The oxygen index (OI) is the minimum percentage of oxygen required to continue flaming combustion of a sample under laboratory condition. Wood samples were initially vacuum treated with the preservative and then were subjected to leaching. Figure 3 showed that the wood treated with flame retardants provided the best results, and moreover, leaching did not considerably change the OI of wood. However, OI of treated sample was affected by leaching procedure, which may be due to the preservatives are not chemically adhered to the wood [38].
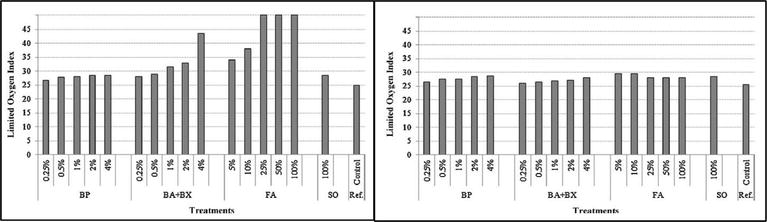
Figure 3.
OI of un-leached and leached flame retardant-treated wood [38].
Disodium octaborate tetrahydrate (Na2B8O13.4H2O) in waterborne paint treatment provides better fire retardant properties of Scot pine wood. The properties are further improved by tannin-based wood preservative solutions [39]. Further, the effect of wood preservative loading on the OI of fir wood was investigated. Wood preservatives have been widely used to extend the service life of wood. Study indicated that almost all treated samples showed higher OI values, and moreover, 3% copper-based preservative (Wolmanit-CB) was recommended [40]. The improvement in dimensional stability, durability and fire retardant properties of wood was investigated by using 1,3-dimethylol-4,5-dihydroxyethyleneurea treatment. The Oak wood showed considerable decrease in the pyrolysis temperature, heat release rate, and smoke production when methylolguanylurea phosphate and boric acid flame retardants were incorporated [41]. There is a current contentious toxicity problem with boron compounds, and as such, a need for wood products to move away from boron-based fire retardants is recognized.
8. Phosphorus-based fire retardant coatings
Fire retardant treatment on wood with flame retardants of both nitrogen compounds and phosphoric acid was examined. The fire retardance and endurance of wood were influenced by method of treatments, such as heat pressed treatment and heat dried treatment. The heat pressed treatment method was improved the properties of wood as compared to heat dried treatment. It was found that the flame retardant properties were further improved by the amount and functional reactivity of flame retardants with formaldehyde as in the dicyandiamide-formaldehyde-phosphoric acid or melamine-dicyandiamide-formaldehyde-phosphoric acid system. The endothermic release of water during the condensation of phosphoric acid can also cool the wood and dilute the volatile pyrolysis gases [42]. Similarly, Subyakto et al. also demonstrated the improvement in fire retardant properties of wood by phosphoric acid treatment, preheating and densifying the surface of wood. Trimethylol melamine formaldehyde resin mixed with phosphoric acid was coated on the wood surface, which was preheated and followed by hot pressing. The pressurized impregnation of coating was improved the fire retardancy of wood without reduction in the bending strength [43]. The fire retardancy of white pinewood was improved by treatment with orthophosphoric acid at different concentration. The aqueous solution of thiourea-formaldehyde resin and orthophosphoric acid was impregnated at different concentration for 1 h. It was found that the weight, compressive strength and fire retardant properties were improved after impregnation. However, the water uptake of treated wood was increased in a water soaking test for 168 h [44].
Organophosphorus flame retardant compounds are not often used in commercial wood applications, and some are known to have high volatility. The synergism between phosphorus and nitrogen is also observed in organophosphorus compounds. Rupper et al. investigated the surface chemistry of cellulose treated with fire retardants containing ethyl ester phosphoramidates. Evidence for a condensed-phase action of the fire retardants was found [45]. It was also showed that phosphoramides, when bonded to cellulose, increase the char yield and lower the weight loss rates in comparison to phosphorus pentoxide and amine-treated wood [46]. The modified pine sawdust with alkyl and phenyl chlorophosphorus compounds using pyridine was prepared by Stevens et al. They reported a reduction in the temperature of maximum pyrolysis rate of up to 90°C and an increase in char formation of up to 29%. The efficiency of the phenyl phosphates was favored compared to the alkyl analogues, and the order of effectiveness was, expectedly, attributed to the acidity and thermal stability, that is, phosphate > phosphonate > phosphinate [47]. Further, it was showed that coupled with a copper-based preservative, the impregnation of wood with an organophosphorus fire retardant reduced the FIGRA by a further 15% [48].
In another study, wood was treated with guanidine compounds, such as guanidine dihydrogen phosphate, diguanidine hydrogen phosphate, guanidine carbonate, and guanidine nitrate and analyzed thermal degradation properties by using thermogravimetric analysis. Char yields were increased compared to untreated wood by approximately 60, 55, 20 and 25%, respectively, which effectively demonstrates the synergism by phosphorus and nitrogen based fire retardants [49]. The effect of urea-formaldehyde oligomer reacted nitrogen-phosphorus flame retardant treatment on dimensional stability and the flame retardant properties of wood was investigated. The results showed that both dimensional stability and OI values were improved significantly in flame retardant impregnated wood. The better flame retardant properties of treated wood are due to formation of protective char layer through dehydration of polysaccharides, which terminate both heat and oxygen [50].
Stejskal et al. studied the flame retardant properties of wood coated with polyaniline, and the coating was made in hydrochloric or phosphoric acid solutions in the absence and presence of stabilizers, poly(n-vinylpyrolidone) or colloidal silica. The coated wood showed less mass loss and formation of charcoal layer on the surface when exposed to direct flame or in a furnace temperature at 400–600°C, as compared to uncoated wood. The similar observation was made with polypyrole and poly(1,4-phenylenediamine) as deposition polymers in wood. The soaking of wood in polyaniline colloids was badly affected the flame retardant properties, whereas the reaction between the cellulose fibers and polyaniline was required to enhance the stability of wood at high temperature. This is attributed to the formation of carbonaceous microtubes, which offered the higher stability of wood against flame and heat exposure [51]. Cyclophosphazene is a polymeric material containing both nitrogen and phosphorus, which has wide range of thermal and chemical stability in addition with fire retardant properties. El-Wahab et al. synthesized the three kinds of cyclodiphosh(V)azane compounds (I-III) and physically mixed in polyurethane varnish formulation at different concentration. It was found that OI of wood panels was increased with loadings (Figure 4). They claimed that improvement was mainly due to several factors, the high molecular weight and aromatic cyclophosphazenes containing chlorine, nitrogen and phosphorus that provides superior flame retardant properties. The presence of N-P bonds renders exceptionally thermally stable, and release less toxic and corrosive gases during burning [52].
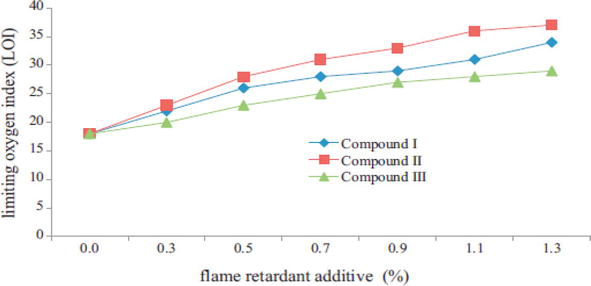
Figure 4.
OI of wood coated with varnish containing cyclodiphosh(V)azane compounds [52].
9. Coatings with synergistic flame retardants
The combinations of silicon and phosphorus have proved popular in the fire retardancy community, in addition to silicon, phosphorus and nitrogen mixtures. The synergism is generally explained as a combination of the individual effects of each of the three additives: phosphorus provides the effective char formation, nitrogen produces non-combustible gases acting as diluents, and silicon offers thermal stability to the substrate by forming a protective layer over the forming char throughout decomposition. The white deposit of silicon dioxide covering the surface of the char will act as a radiant heat shield and help to reduce the rate of oxidation of the char [53]. Grexa and Lubke studied the effect of magnesium hydroxide (MDH), monoammonium phosphate (MAP), aluminum hydroxide (ATH), and boric acid on the flammability of particle board using cone calorimeter at external irradiance of 50 kW/m2. The combination of MDH, MAP and boric acid showed better fire retardant properties, in terms of both heat release rate and smoke production as compared to the same composition contains ATH instead of boric acid. It is expected that while the phosphorus and nitrogen will have behaved synergistically to direct the pathway of pyrolysis toward more char and water and fewer flammable volatiles, the boron present will have become molten to form a glass-like barrier on the surface of the wood, stabilizing the char and enforcing the mass transport barrier. Further, the expandable graphite-based intumescent flame retardants showed lower heat release rate and mass loss rate of particle board when compared to the same loading of ammonium polyphosphate. It was found that the char layer forming flame retardants have a strong effect of flame retardation of wood. The forming char has a distinctive effect in the performance of the material when exposed to external heat in comparison to the same flame retardant without a char layer. They suggested that the intumescent flame retardant system has potential to be used to improve the reaction-to-fire performance of wood [54, 55].
Canosa et al. studied the role of reinforcing fibers on the flammability of intumescent flame retardant coated wood panel. Different film forming materials were chosen to be blended with active ingredients, pigment and several fibers, alumina, carbon, aramid, and glass fibers. Results suggested that chlorinated rubber, phenolic, and epoxy resin showed best performance in thermal conductivity test, 2-foot tunnel, OI and UL94 horizontal-vertical test and also fibers achieved the synergistic effect with intumescent coatings [56]. A 5A-zeolite-treated ammonium polyphosphate (APP) showed better fire performance properties, heat release rate (HRR), total heat released (THR), smoke production rate (SPR), total smoke released (TSR) and fire growth index (FGI) of sawdust board (SB) as compared to APP being used alone (Table 1). Further improvement of fire performance was observed by acid (4-picolinic acid) impregnated 5A-zeolite-treated APP. This can be because acid significantly altered the thermal decomposition and catalytically decomposed to retard the combustion, which promotes the uniform char formation [57].
| Sample | Peak HRR (kW/m2) | Time to peak HRR (s) | Average HRR (kW/m2) | THR (MJ/m2) | SPR (m2/s) | TSR (m2/m2) | FGI (kW/sm2) |
|---|---|---|---|---|---|---|---|
| SB-APP | 122.0 | 40 | 29.2 | 4.4 | 310 | 4.1 | 5.3 |
| SB-APP/5A | 124.8 | 60 | 24.5 | 3.3 | 229 | 2.0 | 3.1 |
| SB-APP/5A | 50.1 | 50 | 8.5 | 1.1 | 215 | 1.9 | 1.4 |
Carbonates and hydrogen carbonates are known to have very high efficiency as gas-phase flame retardants. Potassium carbonate is reported as a compound with a high fire retardant efficiency for wood products. It has a relatively high decomposition temperature (800°C) and serves as a catalyst to the dehydration of wood to increase the production of char, water and CO2. However, the compound is unable to prevent the depolymerization of wood effectively, particularly at high concentrations, and also causes the evolution of CO. As such, the potassium carbonate is only used in low concentrations [58, 59]. The effects of various inorganic salts, Na2WO4, Na2SnO3, Na2MoO4 on the thermal decomposition and fire retardant properties of wood sample were demonstrated. Upon the treatment of these salts, the OI of wood sample was increased, which is caused by an increase in the amount of char on the surface. The activation energies of the samples were also decreased after treatment during both the charring stage and the calcining stage. The flame retardants were shown to be able to catalyze the dehydration reaction, resulting in the formation of more H2O, CO2, and char, but less flammable vapors like levoglucosan and levoglucose [60].
The effects of pressure and microwave heating duration on the flammability of ammonium polyphosphate (APP) impregnated wood samples were studied. The flame retardant properties, such as peak heat release rate, total heat released and total smoke released, were measured for samples of pretreated and untreated with microwave and characterized by cone calorimeter. It was found that the treated wood showed better flame retardant properties, and moreover, the microwave pretreatment of wood can also increase the fire retardant properties of APP impregnated wood [61]. Wang et al. found that the expanded vermiculite treatments improve the flame retardant properties of plywood. Results showed that expanded vermiculite treatments increase the OI values of wood and at the same time decrease the thermal activation energy at the maximum degradation process. The increase in OI is due to the formation of protective layer on the surface of wood sample [62].
10. Intumescent fire retardant coatings
The intumescent coating of either pigmented or clear was introduced to the fire retardation of wood-based products. Intumescent coatings swell and char when exposed to heat, giving carbonaceous foam that insulates the surface from the fire. The char layer is also responsible for the limitation of oxygen diffusion and the reduction of the volatilization of the fuel in order to prevent the continuation of the combustion cycle. Intumescence resulted from the application of flame retardant coating systems to timber can reduce the char formation and heat buildup and also delay the onset of combustion of a wood [63]. Gardner and Thomson have studied the flammability of forest products, including sawn boards, plywood, hardboards and particleboard as per ASTM E906–Standard test method for heat and visible smoke release rates for materials and products. The intumescent flame retardant was used to treat the plywood by pressure impregnation. The ignition time of treated sawn board was not increased with density when exposed to heat fluxes of 20 and 40 kW/m2. However, the ignition times of plywood were increased by pressure impregnated flame retardants. The heat release properties of both samples were reduced by flame retardant coatings, which is dependent on the exposed heat flux [64].
An intumescent fire-retardant coating based on unsaturated polyester and epoxy resin was prepared by using ammonium polyphosphate, pentaerythritol, melamine, and expandable graphite, and studied the fire endurance performance of wooden board. Results showed that 2 mm film thickness of intumescent coating provides excellent fire endurance time [65]. Chou et al. investigated the usefulness of an artificial graphite powder and a sericrite (Al4(OH)4(KAl-Si3O10)2), and a mixture of the two on plywood. The intumescent fire retardant coating formulation contains 19.8% of the flame retardant, 15% of the dehydration agent, 18% of the foaming agent, 7.2% of the resin binder and 40% of the solvent which was prepared and applied to the surface of plywood. They showed that when sericrite was in excess of 75% in the fire retardant composition, the mixture obtained the lowest flammability grade possible in Taiwan Standard CNS 7614. Furthermore, for sericrite to be effective in inhibiting combustion and also carbonizing agents, such as graphite powder, are not required [66]. The burning behavior of intumescent coating-treated flax board was studied by cone calorimeter and single burning item (SBI) test. Intumescent coating provides significant increase in ignition time and decrease in heat release rate of flax board. It was also confirmed that experimental results are comparable to numerical predictions, factional factor, the ratio of the heat flux at the interface of the intumescent surface and the char layer of flax board to the surface heat flux when the absence of intumescent coating layer, based on analytical solutions for charring materials and burning rates in SBI tests [67]. Intumescent coatings are commonly used for protecting steel structures. Their application on wood has also been studied, and some commercial products intended for wood are available. At present, however, fire protection of wooden and wood-based products with intumescent coatings is not widely employed.
11. Transparent fire retardant coatings
The transparent ultraviolet (UV) curable intumescent fire retardant coating on wood was prepared by using cyclotriphosphazene as a flame retardant. A series of (2-hydroxyethylmethacrylate)(n-propoxy)cyclotriphosphazenes were prepared by the reaction of N3P3Cl6 with n-propanol and 2-hydroxyethylmethacrylate sequentially in the appropriate solvents. It was observed that phosphazene-based compounds showed better fire retardant properties of wood and without affecting the aesthetic appearance of wood structure [68]. Chen et al. prepared the dual cured (UV-radiation and moisture) flame retardant coatings based on silicone and phosphate modified acrylates. The oligomer was made by reacting 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide and 3-glycidoxypropyltriemethoxysilane, and reacted with 2,4-toluene diisocyanate and 2-hydroxyethyl acrylate. The dual cured film showed high-flame retardance, which is attributed to the synergistic effect of phosphorus-silicon and phosphorus-nitrogen [69]. Shi and Wang synthesized the transparent intumescent flame retardant coatings from epoxy/phosphate flame retardant and amino resins. Five types of flame retardants were prepared by a two-step reaction using 1-oxo-4-hydroxymethyl-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane, polyphosphoric acid, bisphenol-A epoxy resin and 1,3-butanediol diglycidyl ether with different proportions. The structure of flame retardant was confirmed by nuclear magnetic resonance (1H-NMR) and Fourier transforms infrared (FTIR) spectroscopy. It was found that the fire retardant properties of plywood were significantly improved by intumescence and also enable to maintain the visibility of the surface features of wood [70].
12. Nanocomposite coatings
Nanoparticles have recently been used to prepare the nanocomposites for the improvement in fire retardant properties. The major concern of these materials is dispersion. The surface modification is essential for nanoparticles to achieve better compatible and homogeneous dispersion. The necessary loading of nanoparticles is usually lower than for their micron-sized filler counterparts which are an enormous advantage industrially and economically. Clay nanopowder composed of montmorillonite and cellulose nanofibers which is used as a transparent fire retardant coating for wood was demonstrated. Fire performance was assessed by cone calorimeter at irradiance of 35 kW/m2. It was found that nanopowder coated wood showed a strong increase in time to ignition, and a 33% total heat release was reduced along with the 46% reduction in maximum average heat emission rate. Both thermal shielding and gas barrier functions contribute to delayed thermal degradation of wood and delayed emission of volatile combustible gases [71]. Hassan et al. prepared the flame retardant intumescent polyurethane coatings for wood products. The limitation of intumescent additives, such as incompatibility and loading issue, was addressed by using butyl acrylate and montmorillonite clay. They found that the flame retardant properties of wood were improved by addition of acrylate and nanoclay using cone calorimeter [72]. Giudice and Pereyra showed through the use of oxygen index and a two-foot tunnel tests, (ASTM E84 2013—the regulatory test for interior flammability of building materials in the United States), the silica nanoparticle-treated wood provides several significant advantages: high fire retardancy, low thermal expansion, reduced smoke production, and low cost [73]. The reaction-to-fire properties of titanium dioxide (TiO2) and/or clay nanoparticles coated spruce wood were investigated by Fufa et al. using a small-scale cone calorimeter. They found that the negative results of reaction-to-fire performance along with water vapor permeability were observed on specimens treated with TiO2 and/or clay nanoparticles treatments [74]. Chuang et al. have investigated the fire performance of intumescent coated plywood with the addition of commercial organoclays (Cloisite 30B, Cloisite 10A, and Cloisite 15A) at different loadings, 1, 3, 5, and 10%. According to the cone calorimeter test, compared to uncoated plywood, the intumescent coating exhibited lower peak heat release rate (peak HRR) and extend the time to reach peak HRR. Further improvement was observed with the incorporation of organoclays; however, the type and amount of organoclay are very important. For instance, 3% Cloisite 30B and 5% Cloisite 10A displayed better fire retardancy as compared to other loadings of the clay. It was demonstrated that adding organoclay extends the survival duration of the phosphocarbonaceous char structures, which is confirmed by spectroscopic studies [75].
Zhang et al. studied the ignition and burning behavior of intumescent coating and sepiolite nanoparticles applied on flaxboard using cone calorimeter, single burning item (SBI) test, and reduced scale (one-third) ISO room test. Both cone calorimeter and SBI test represent an open and well-ventilated condition, whereas reduced ISO room test is a fire in a confined space and represents a more realistic burning condition in most compartment fires. Intumescent coatings effectively delaying the ignition and mass loss rate in both cone calorimeter and SBI tests. Further improvement was observed in addition of nanoparticles. This because of intumescent coating forms carbonaceous char on the surface, which acts as a thermal and physical barrier preventing heat, mass and gas transfer, and further improvement is due to an increase in the thermal stability of the char as confirmed by the thermogravimteric analysis. In addition, it was also confirmed that intumescent coatings do not cause an increase of the toxic gases, and ventilation plays a vital role in the development of the fire [76].
The high thermal conductivity of nanosilver coatings was tested to improve heat transfer in wood and enhance fire retardant performance. Nanosilver treatment clearly showed potential in improving some of the fire retarding properties in solid wood products. It can be observed that coating may delay thermal degradation and carbonization by reducing the accumulation of heat that is rapidly transferred [77]. Nano-wollastonite is demonstrated as multifunctional additive in wood. Wollastonite nanofibers were also reported the same group to improve durability and fire retardant properties of poplar wood and solid wood composites. It was found that both fire retardant and dimensional stability were improved at 10% nano-wollastonite impregnated wood. As a mineral material of wollastonite, acts as an impermeable physical barrier toward the penetration of flames into the wood structure [78]. The synthesis of hexagonal boron nitride nanosheets was demonstrated through a facile shear force liquid phase exfoliation method and used as a binder free oxidation and fire retardant wood coatings. Because of intrinsic low thermal diffusivity and thermal effusivity, nanosheet coatings showed an excellent fire retardation and oxidation resistance up to 900°C [79]. The effect of organically modified alpha-zirconium phosphate (OZrP) on the thermal and fire retardant properties UV curable system was studied. The flame retardant coating system comprises of phenyl di(acryloyloxyethyl)phosphate (PDHA), triglycidylisocyanurate acrylate (TGICA) and 2-phenoxyethyl acrylate (PHEA). Results showed that addition of 0.5 wt% OZrP, the peak heat release rate and total heat of combustion were reduced significantly. This is because of the effective char formation and improvement of anti-oxidation performance of the coating [80].
13. Sol-gel method
In recent years, sol-gel processes have also become recognized for the purposes of incorporating fire retardants into products. The process comprises hydrolysis and condensation reactions that lead to the formation of inorganic or organic-inorganic hybrid coatings. This technique is well documented for different polymers. Giudice et al. synthesized the polysiloxanes in wood pores by sol-gel process using aminopropylmethyldiethoxysilane, aminopropyltriethoxysilane and a mixture of both (50/50 ratio), and then, impregnated panels were subjected to 2-foot tunnel test (flame spread index, panel consumption, and smoke density). Impregnation process was carried out at 40–50°C in an autoclave and controlling the operating conditions for achieving different weight gains. It was shown by the authors that aminopropyltriethoxysilane-treated wood sample showed best fire retardant efficiency. This is because of more reactivity of alkoxide, which forms hybrid structure [81]. Another study showed that the transparent fire retardant coating for wood (pine and larch) was prepared by a sol-gel method using vinyl functionalized zirconium oxy-clusters copolymerized with vinyl trimethoxysilane. Results showed that coating has improved the fire retardant properties and also without affecting the macroscopic appearance of wood surface [82].
14. Fire resistance of timber
The use of timber components in the loading structure in a building relies on fire engineering design to ensure that the building can retain its structural integrity for sufficient time either for building occupants to be evacuated, or for the fire to be extinguished. In construction using large cross-section timber members, like cross-laminated timber, this may be done by assuming a rate at which the timber chars and therefore the cross-section of timber remaining after a given time [83]. The strength and stiffness of timber both reduce at lower temperatures than steel and concrete. For example, timber’s strength is reduced by more than 50% at 100°C, compared with that at 20°C [84]. Timber structural members may still perform well at high temperatures in comparison with steel, however, since the char layer can act to insulate the material within, whereas the high thermal conductivity of steel means that the complete section quickly heats up. Where steel is used to connect timber elements, heat can be quickly conducted through the connectors, degrading the strength and stiffness of the wood around them. The behavior of timber in fire is fundamentally different to steel and reinforced concrete; however, since it is combustible, research groups have identified that the key research needs to be addressed for the next generation of large timber buildings [85, 86]. They address the performance of systems with various levels of encapsulation, the effect of flame spread due to a combustible structural material, and the fire performance of connections. Another potential use for coatings is to increase the fire resistance of structural timbers. It was showed that significant increases in fire resistance can be achieved by using fire resistant coatings. This concept should be pursued by the timber industry, as it would be possible to improve the fire resistance of structural timbers in old buildings that are being remodeled. The application of a fire resistive coating would be simpler and cheaper than cladding the members in fire resistive board materials, or replacing the timber member with a concrete or steel member.